Analysis of Catalysts
Taking Control over Chemical Reactions
Catalysis is the effect that certain substances, so-called catalysts, are able to specifically influence the kinetics of a chemical reaction. Catalysts reduce the activation energy of the respective chemical reaction without being consumed in it. Often heterogeneous catalysts are used where the catalyst consists of a solid material. This material itself can be active in the reaction, as is the case with e.g. zeolites, or the catalyst can have an active phase dispersed on a support. With the latter method small amounts of expensive noble metals are needed to obtain a large surface area of these metals. Examples of these supported catalysts are Pd/C hydrogenation catalysts or automotive catalysts containing Pt and Rh on Ceria.
Heterogeneous catalytic reactions take place at the outermost atomic layer of a catalyst. It is therefore not surprising that quantitative, surface analytical methods such as Low Energy Ion Scattering Spectroscopy (LEIS), time-of-flight secondary ion mass spectrometry (ToF-SIMS) or photoelectron spectroscopy (XPS, ESCA) are used in the development of catalysts and in the search for the causes of failure of catalysts. Ideally, catalysts emerge unchanged from a chemical reaction. However, catalysts can be deactivated by a variety of mechanisms and they can be poisoned by specific contamination. In that case, the cause should be clarified. The analysis of catalysts has been part of our daily work in the Tascon laboratory for over 20 years. The following example shows the analysis of a catalyst in our laboratory.
Analysis of the catalyst regeneration using LEIS
The present example shows how the activity of a 3-way catalytic converter is negatively influenced by "cold start" conditions. The catalyst consists of platinum and rhodium on an oxide base.
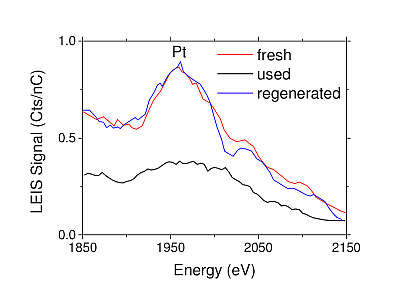
The LEIS spectrum of the surface of a new catalyst ("fresh" in red) shows a clear platinum peak. Since LEIS is sensitive to the outermost atomic layer of a material, this means that the Pt can be in contact with the reactants. When the catalyst is used under cold start conditions, this Pt peak is no longer visible, since the platinum surface is covered with coke (carbon). The platinum is therefore no longer available for a catalytic reaction. The carbon can be removed by a regeneration step. This leads to the exposure of the platinum surface ("regenerated") and the catalytic activity is restored. In addition to the analyses shown here, it is also possible in the Tascon laboratory to analyze and identify substances that occupy the catalyst surface. The ToF-SIMS analysis is suitable for this purpose due to its excellent screening properties.
Tascon - your partner in the analysis of catalysts
We at Tascon are your competent laboratory partner in the field of catalyst characterization and support you both in error analysis in the field of catalysis and in the development of catalysts. We can look back on more than 20 years of expertise in the field of surface analysis. If you are interested in our service, please contact us. The experts from our team will advise you without obligation. We look forward to your inquiry.
Get in touch. Contact one of our analytical professionals:
(845)-352-1220

