Analysis of the Oxidation State
Type of chemical bond and determination of average bond states using XPS
Macroscopic material properties (e.g. color, conductivity, adhesion, corrosion resistance, wettability, ...) are significantly influenced by the oxidation states and binding states of the elements present on a solid surface. It is therefore often of great interest for many branches of industry to analyze functional groups and end groups as well as the oxidation levels of the atoms contained on surfaces. XPS offers the possibility to examine these chemical bond states in detail. In this way, conclusions can be drawn about the valence of the individual elements and, hence, about their oxidation state. It is possible to examine substrates such as glass, ceramic, metal or plastic and others. In addition to the qualitative analysis of materials, the quantitative analysis of materials is also possible. Different layers consisting of e.g. fluorides, carbides, oxides or nitrides can be quantified reliably in our laboratory. The following example shows how XPS can be used to determine the oxidation state.
XPS - Ideal technique for determining oxidation states
A molybdenum sulfide powder (MoS2) used as a lubricant was analyzed with the aid of XPS for the quantitative determination of oxidized Mo components and the average oxidation state.  The identification and quantification of the elements present on the powder surface was first carried out using an XPS overview spectrum (for results see Table 1).
The identification and quantification of the elements present on the powder surface was first carried out using an XPS overview spectrum (for results see Table 1).
For the detected elements, energy spectra with high resolution were also acquired in the Tascon laboratory to quantitatively evaluate the existing binding states. The degree of oxidation was determined by evaluating the S2p and Mo3d spectra (see Figure 1a and b). The S2p spectrum initially shows a characteristic S2p doublet, which, due to its energetic position, shows the binding of the complete sulfur in sulfidic form as MoS2. Based on the Mo3d spectrum, molybdenum can be detected in two oxidation states (MoS2 and MoO3).
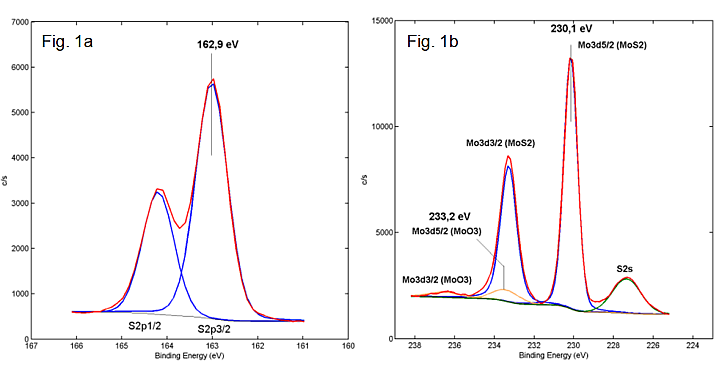
In addition to the different Mo oxidation states, an S2s signal can also be seen in Figure 1b, which again indicates a MoS2 bond of the sulfur. The corresponding binding states can be quantified via the relative peak areas of the Mo3d signals of the MoS2 or MoO3 species. If one takes into account the surface C and partial O contamination of the MoS2 powder already listed in table 1, this leads to the quantitative assignment to the individual components listed in table 2:
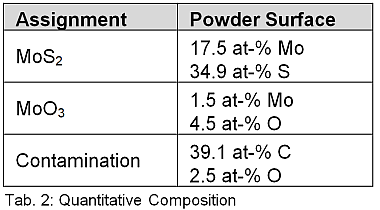
The evaluations of the Mo3d spectrum make it possible to determine the MoO3 portion in MoS2. According to this, approximately 7.9% of the molybdenum is present as Mo (VI) O3 in the powder.
Tascon - your partner for the investigation of oxidation and binding states
If you are interested in further details, contact Tascon’s experts in the laboratory for the analysis and investigation of oxidation states on any surface. Contact us and let us advise you personally and without obligation.
Get in touch. Contact one of our analytical professionals:
(845)-352-1220

