Analysis of Nanoparticles
Diverse analysis aspects for diverse applications
Nanoparticles have become an indispensable part of daily use; they can be found in a multitude of applications today. Gold nanoparticles are used in cosmetics, silver nanoparticles in antibacterial plasters, titanium dioxide nanoparticles in packaging or organic nanoparticles for medical applications. Since the applications of nanoparticles are diverse, so are the issues involved in the analysis of nanoparticles. The following analysis aspects are often in the foreground: Analysis of the particle shape, analysis of the particle surface or the investigation of the chemical composition of nanoparticles, e.g. Core-shell nanoparticles. Depending on the question, special techniques such as ToF-SIMS, XPS, LEIS or SEM are used for the analysis of nanoparticles in the Tascon laboratory. The following example shows a typical application of XPS in the analysis of nanoparticles that were developed for pharmaceutical use:
Spray instead of syringe
XPS analysis to support the development of new dosage forms
The oral intake of insulin is being developed as an alternative to the widespread injection of this hormone. Biodegradable nanoparticles are suitable as insulin carriers, which envelop the insulin and only release it after it has entered the bloodstream. The quality of the nano-shell and the amount of the encapsulated hormone are of decisive importance for the effectiveness of oral intake. For this reason, surface-sensitive XPS tests in our laboratory were combined with volume analytical methods in order to obtain qualitative and quantitative statements about the quality of the insulin encapsulation.
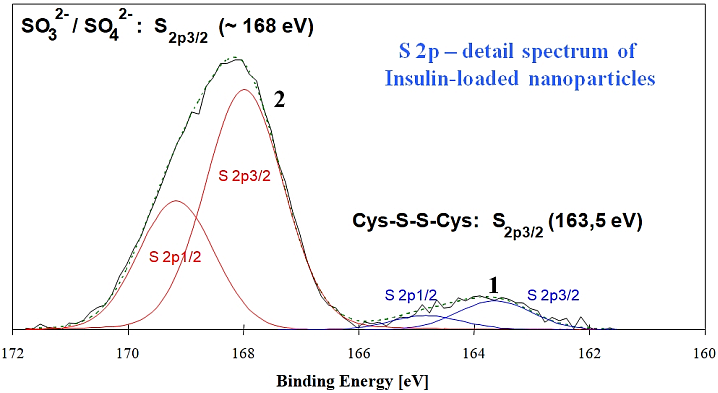
The XPS spectra for insulin and the unloaded nanoparticles are very similar. These show signals of the main elements C, O and N. Since XPS is sensitive to the chemical surrounding of the element, there are differences in the peak positions for the different S species. This means that in the XPS spectrum, insulin can be differentiated from the nanoparticles by its characteristic disulfide bridge (Cys-S-S-Cys), as the S2p detailed spectrum in the picture above shows. Because of the disulfide bridges, the insulin has a signal with an S2p3/2 binding energy (BE) of 163.5 eV. The SO32- / SO42- species (BE approx. 168 eV), that were also detected, can be assigned to a sulfur component present on the nanoparticles.
With XPS, a quantitative detection of the insulin in the top 10 nm of a nanoparticle is possible due to the information depth of the process. A comparison with the results of a determination of the bulk insulin content enables an estimation of the surface area of the hormone.
|
Particle |
Insulin fraction in the bulk of the nanoparticles |
Insulin fraction at the surface of the nanoparticles |
|
Batch 1 Batch 1, washed |
13.6 % not determined |
5.0 % 3.6 % |
|
Batch 2 Batch 2, washed |
13.3 % not determined |
21.0 % 5.3 % |
Table 1: Insulin content in the non-washed nanoparticles and on their surface before and after washing
Table 1 shows the results of two batches of nanoparticles. While the total insulin content of both batches is almost identical, the XPS data show a significantly higher insulin content on the surface of batch # 2. In addition, the insulin content of this batch can be significantly reduced by washing. This indicates improper or incomplete encapsulation of the hormone. These nanoparticles are then unsuitable as carriers of insulin for oral absorption.
Tascon - Laboratory for the Analysis of Nanoparticles
Does your company work with nanoparticles and do you need our support for the analysis of nanoparticles? Then simply contact Tascon and let us advise you without obligation. Our competent and friendly team is happy to be there for you. We look forward to your inquiry.
Get in touch. Contact one of our analytical professionals:
(845)-352-1220

